Introduction to semiconductors
what are the semiconductors? How they are used for designing different electronic components? The best conductors (silver, copper, and gold) have one valence electron, though the best covers have eight valence electrons. A semiconductor is a component with electrical properties between those of a conductor and those of insulators. As you would expect, the best semiconductors have four valence electrons.
Germanium
Germanium is an illustration of a semiconductor. It has four electrons in the valence circle. Numerous years prior, germanium was the solitary material appropriate for making semiconductor gadgets. Be that as it may, these germanium gadgets had a deadly flaw (their extreme converse current, examined in a later segment) that specialists proved unable survive. At last, another semiconductor named silicon got commonsense also, made germanium old in most electronic applications.
Silicon
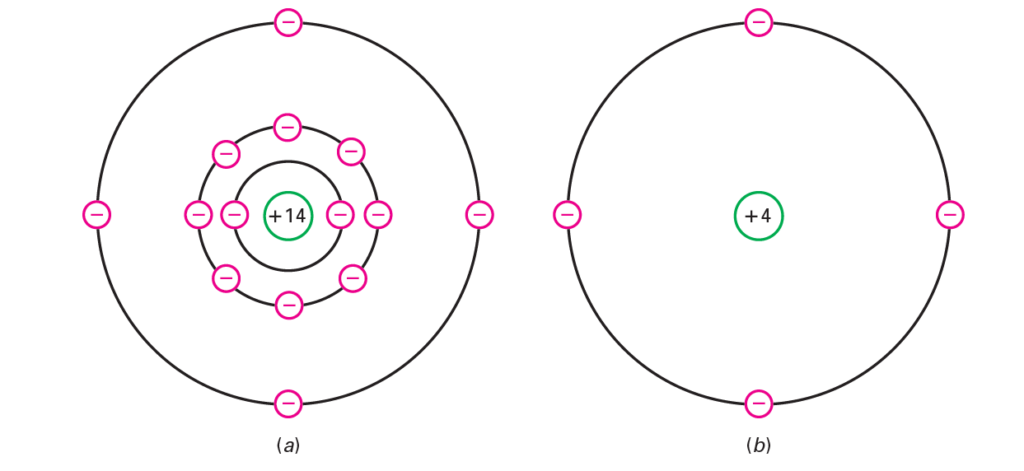
Close to oxygen, silicon is the most bountiful component on the earth. In any case, there were certain refining issues that forestalled the utilization of silicon in the beginning of semiconductors. When these issues were tackled, the upsides of silicon (examined afterward) quickly settled on it the semiconductor of decision. Without it, present day gadgets, interchanges, and PCs would be incomprehensible. A disconnected silicon iota has 14 protons and 14 electrons. As demonstrated in Fig. below, the first circle contains two electrons and the subsequent circle contains eight electrons. The four excess electrons are in the valence circle. In Fig. below, the center has a net charge of 14 since it contains 14 protons in the core and 10 electrons in the first two circles. Figure below shows the center graph of a silicon iota. The four valence electrons reveal to us that silicon is a semiconductor.
Silicon Crystals
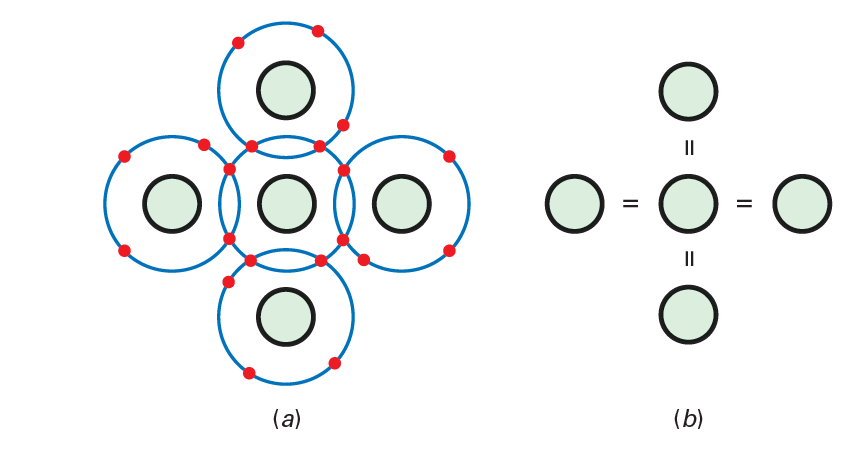
At the point when silicon molecules join to frame a strong, they organize themselves into an systematic example called a precious stone. Every silicon molecule imparts its electrons to four adjoining molecules so as to have eight electrons in its valence circle. For example, Fig. below shows a focal particle with four neighbors. The concealed circles speak to the silicon centers. Albeit the focal iota initially had four electrons in its valence circle, it presently has eight.
What are Covalent Bonds?
Each adjoining particle imparts an electron to the focal iota. Along these lines, the focal molecule has four extra electrons, giving it a sum of eight electrons in the valence circle. The electrons at this point don’t have a place with any single particle. Every focal molecule and its neighbors share the electrons. A similar thought is valid for all the other silicon particles. As such, every particle inside a silicon precious stone has four neighbors. In Fig. below , each center has a charge of 14. Take a gander at the focal center and the one to one side. These two centers pull in the pair of electrons between them with equivalent and inverse power. This pulling in inverse ways is the thing that holds the silicon molecules together. The thought is like back-and-forth groups pulling on a rope. However long the two groups pull with equivalent and inverse power, they remain reinforced together.
Since each shared electron in Fig. below is being pulled in inverse ways, the electron turns into a connection between the contrary centers. We call this sort of compound bond a covalent bond. Figure below is a more straightforward approach to show the idea of the covalent bonds. In a silicon gem, there are billions of silicon particles, each with eight valence electrons. These valence electrons are the covalent bonds that hold the gem together—that give it strength.
Valence Saturation
Every particle in a silicon precious stone has eight electrons in its valence circle. These eight electrons produce a compound security that outcomes in a strong piece of silicon material. Nobody is very certain why the external circle of all components has an inclination toward having eight electrons. At the point when eight electrons don’t exist normally in an component, there is by all accounts a propensity for the component to join and share electrons with different particles in order to have eight electrons in the external circle. There are progressed conditions in physical science that halfway clarify why eight electrons produce compound soundness in various materials, however nobody knows the motivation behind why the number eight is so extraordinary. It is one of those laws like the law of gravity, Coulomb’s law, and different laws that we notice yet can’t completely clarify.
At the point when the valence circle has eight electrons, it is immersed in light of the fact that no more electrons can fi t into this circle. Expressed as a law:
valence saturation n=8
In words, the valence circle can hold close to eight electrons. Besides, the eight valence electrons are called bound electrons since they are firmly held by the molecules. In light of these bound electrons, a silicon precious stone is right around a ideal encasing at room temperature, roughly 25°C.
The Hole
The encompassing temperature is the temperature of the encompassing air. At the point when the encompassing temperature is above outright zero (2273°C), the warmth energy in this air makes the particles in a silicon precious stone vibrate. The higher the encompassing temperature, the more grounded the mechanical vibrations become. At the point when you get a warm object, the glow you feel is the impact of the vibrating iotas. In a silicon precious stone, the vibrations of the particles can once in a while remove an electron from the valence circle. At the point when this occurs, the delivered electron gains enough energy to go into a bigger circle, as demonstrated in Fig. below. In this bigger circle, the electron is a free electron.
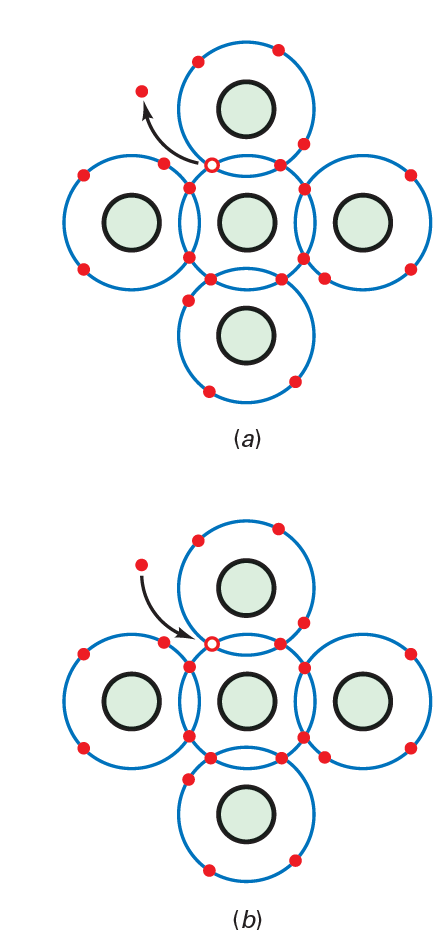
Yet, that is not all. The takeoff of the electron makes an opening in the valence circle called an opening (see above figure). This opening carries on like a positive charge since the deficiency of the electron creates a positive particle. The opening will pull in and catch any electron in the quick region. The presence of openings is the basic contrast among conductors and semiconductors. Openings empower semiconductors to do a wide range of things that are inconceivable with conductors.
At room temperature, nuclear power delivers a couple of openings and free electrons. To build the quantity of openings and free electrons, it is important to dope the precious stone. More is said about this in a later area.
Recombination and Lifetime
In an unadulterated silicon precious stone, warm (heat) energy makes an equivalent number of free electrons and openings. The free electrons move arbitrarily all through the precious stone. a Periodically, a free electron will move toward an opening, vibe its fascination, and fall into it. Recombination is the converging of a free electron and an opening .
The measure of time between the creation and vanishing of a free electron is known as the lifetime. It shifts from a couple of nanoseconds to a few microseconds, contingent upon how amazing the precious stone is and different elements.
Also read here: