Introduction to Millikan’s Oil Droplet Method
What is Milikan’s Oil Droplet Method? The charge of the electron is measured using the classic technique of Millikan. Measurements are made of the rise and fall times of oil droplet illuminated by light from a Helium-Neon laser. A radioactive source is used to enhance the probability that a given drop will change its charge during observation. The emphasis of the experiment is to make an accurate measurement with a full analysis of statistical and systematic errors.
INTRODUCTION:
Starting in 1908, while a professor at the University of Chicago, Robert A. Millikan worked on an oil-drop experiment in which he measured the charge on a single electron. J. J. Thomson had already discovered the charge-to-mass ratio of the electron. However, the actual charge and mass values were unknown. Therefore, if one of these two values were to be discovered, the other could easily be calculated. Millikan and his then graduate student Harvey Fletcher used the oil-drop experiment to measure the charge of the electron (as well as the electron mass, and Avogadro’s number, since their relation to the electron charge was known).Professor Millikan took sole credit, in return for Harvey Fletcher claiming full authorship on a related result for his dissertation.
Millikan oil-drop experiment, first direct and compelling measurement of the electric charge of a single electron. It was performed originally in 1909 by the American physicist Robert A. Millikan and Harvey Fletcher, who devised a straightforward method of measuring the minute electric charge that is present on many of the droplets in an oil mist. The force on any electric charge in an electric field is equal to the product of the charge and the electric field. Millikan was able to measure both the amount of electric force and magnitude of electric field on the tiny charge of an isolated oil droplet and from the data determine the magnitude of the charge itself.
The elementary charge is one of the fundamental physical constants, and accurate knowledge of its value is of great importance. His experiment measured the force on tiny charged droplets of oil suspended against gravity between two metal electrodes. Knowing the electric field, the charge on the droplet could be determined. Repeating the experiment for many droplets, Millikan showed that the results could be explained as integer multiples of a common value (1.592 × 10−19 coulomb), which is the charge of a single electron. That this is somewhat lower than the modern value of 1.6022 x 10−19 coulomb is probably due to Millikan’s use of an inaccurate value for the viscosity of air.
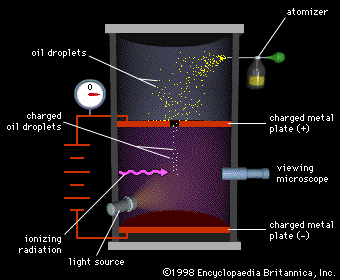
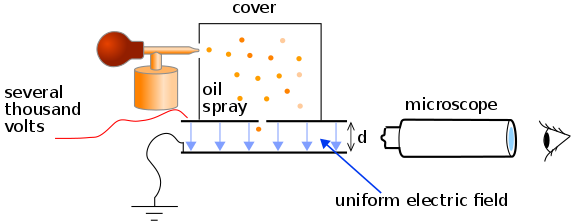
METHODOLOGY:
- The apparatus consists of a metallic chamber. It has two parts. The chamber is filled with air, the pressure of which can be adjusted by a vacuum pump.
- There are two electrodes (parallel pates) P and P’ inside the chamber (container C) to avoid disturbances due to air currents. These electrodes are used to generate an electrical field in the space between the electrodes. The separation between the plates is d.
- The upper plate has a small hole H, as shown in the figure. A voltage V is applied to the plates due to which the electric field E is setup between the plates. The magnitude of its value is E=V/d.
- An atomizer is used for spraying oil drops into the containers through a nozzle. The oil drop gets charged because of the friction present between the walls of atomizer and oil drops.
- Ordinary oil was not used for this experiment as it would evaporate from the heat of the lamp so it could cause an error in the Milliken’s Oil Drop Experiment. Therefore, the most commonly used oil is a vacuum that is under pressure.
- These oil drops are very small and are actually in the form of mist. A few droplets passes through the hole in the upper plate and into the region between the charged plates, where the path of downward motions of these droplets are observed through a microscope
- The space between the plates is illuminated by the light coming from a light source. So the droplet, when illuminated perpendicularly to the direction of view, appears in the microscope as a bright speck against a dark background.
- Gravity attracts the oil in a downward direction and the electric field pushes the charge upward. The strength of the electric field is regulated so that the oil droplet reaches an equilibrium position with gravity.
- The charge over the droplet is calculated at equilibrium, which is dependent on the strength of the electric field and mass of droplet.
DERIVATION FOR CHARGE ON ELECTRON:
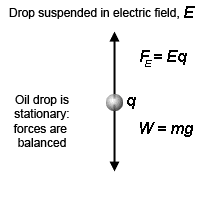
A given droplet between the two plates can be suspended in air if the gravitational force Fg acting on the drop is equal to electrical force Fe. The Fg can be equal to Fe by adjusting the voltage. In this case, we can write
Fe = Fg
Where, Fg = w =mg
Also, E = F , which can be written as, Fe = qE.
So,
qE = mg
If V is the voltage between the plates for this setting, then
E = V/d
We may write as,
Which is the equation to find charge on an electron.
In order to determine the mass m of the droplet, the electric field between the plates is switched off. The droplet falls under the action of gravity through air. It attains terminal speed , almost at the instant the electric field is switched off. Its terminal speed , is determined by timing the fall of the droplet over a measured distance. Since the drag force F due to air acting upon the droplet when it is falling with constant terminal speed is equal to its weight.
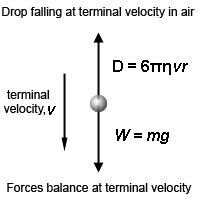
Hence, using Stokes’s law,
Fd = Fg
6πηr = mg
Where, r is the radius of the droplet and η is the “coefficient of viscosity” for air. If ρ is the density of the droplet, then mass of the droplet will be
Mass = Density(Volume)
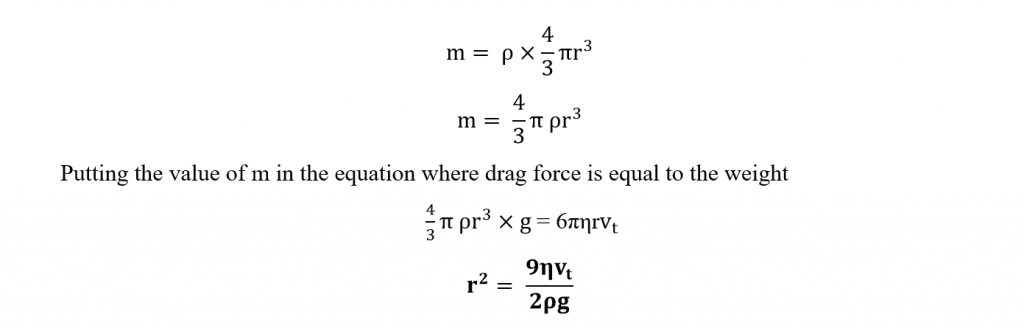
Knowing the value of r, the mass m can be calculated. This value of m is substituted in the equation of q, to get the value of charge q on the droplet.
By changing the strength of electrical field, Millikan found that the charge on each droplet was different. The smallest charge which he found was 1.592 × 10−19 coulomb, which is very close to the recent value of 1.6022 x 10−19 coulomb. This smallest charge on any droplet is the charge of one electron. The other drops having more than one electron on them, have double or triple the amount of this charge. The charge present on an electron is the smallest charge of electricity that has been measured so far.
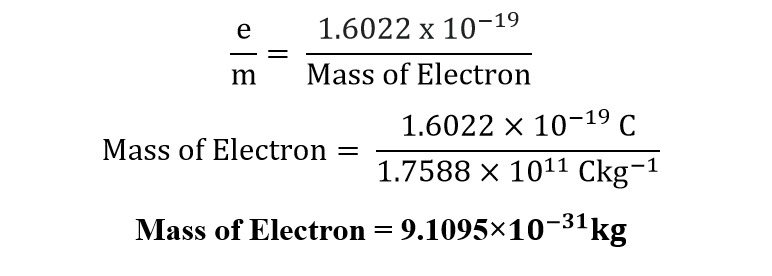
- MASS OF ELECTRON:
As the value of charge on an electron is 1.6022 x 10−19 coulomb, while e/m value of electron was given by J.J Thomson, which was 1.7588 coulombs per kg. So,
CONCLUSION:
- The charge over any oil droplet is always an integral value of e (1.6 x 10-19C). Hence, the conclusion of Millikan’s Oil Drop Experiment is that the charge is said to be quantized, i.e. the charge on any particle will always be an integral multiple of ‘e’.
- Millikan’s oil drop experiment measured the charge of an electron. Before this experiment, existence of subatomic particles was not universally accepted.
Also read here
how to design an advanced 1-bit Arithmetic Logic Unit (ALU) with Hybrid Memristor CMOS Architecture?