What is a Battery and Battery Life?
what is a battery and battery life? Around 1800, Alessandro Volta detailed the aftereffects of a trial where he had the option to make power by heaping plates of zinc and copper isolated by felt spacers absorbed salt water. With this innovation, Volta demonstrated that power could be created artificially and exposed the pervasive hypothesis that power was created exclusively by living creatures. Another creation was born: the battery. A battery is a device that is capable of generating electricity like a power supply.
Define Battery Life
Batteries are electrochemical devices made by implanting two diverse metallic plates into a third one. This third one, the electrolyte, is dependable, extensively talking, for making a compound response so that one of the components closes with abundance of electrons (negative charges) and the other one with an abundance of positive charges – as such, to make an unevenness of charges between the plates. To sum up how substance energy is changed over into electrical energy, let us take an illustration of a normal vehicle battery: one plate of unadulterated lead and another made of lead dioxide both inundated in an answer of sulfuric corrosive weakened with water. Synthetic response makes the lead become adversely charged and the lead dioxide to turn out to be emphatically charged.
The component with abundance of electrons will be the negative shaft (cathode) and the other component will be the positive post (anode). This distinction in the quantity of electrons between the shafts is designated “contrast in electric potential” or prominently, “voltage.” Any component, similar to a light, for instance, associated between the posts, will give a way to the overabundance of electrons of the lead component to relocate to the lead dioxide, which needs electrons. As the response occurs, the majority of the sulfuric corrosive in the electrolyte is devoured and used to change over the two plates into lead sulfate, and the electrolyte transforms into, for the most part, customary water. Sooner or later, every one of the three components are electrically adjusted, current quits streaming, what’s more, the battery kicks the bucket.
In earlier days, voltage was named as electromotive (emf) force since it was supposed to be kind of pressure that causes the current to force through a conductor. It was said that electrons flow from negative to positive plate with a slow speed and the direction of electric field was same as that of it. While the current flows in the opposite direction i.e., from high potential towards the low potential.
The way toward charging the battery will apply energy to the plates and electrolyte to turn around the reaction, making the plates to be lead dioxide and lead and the electrolyte sulfuric corrosive and water, once more.
All power supplies have limited value of current and voltages they can supply. There is a direct relation between the current and number of charges that a power supply can move over time.
What Happens When Two Batteries are Connected in Series?
Consider the following figure where two batteries of same type are connected in series. The positive terminal of the battery is connected to the negative terminal of the battery and wires are connected on the other ends.
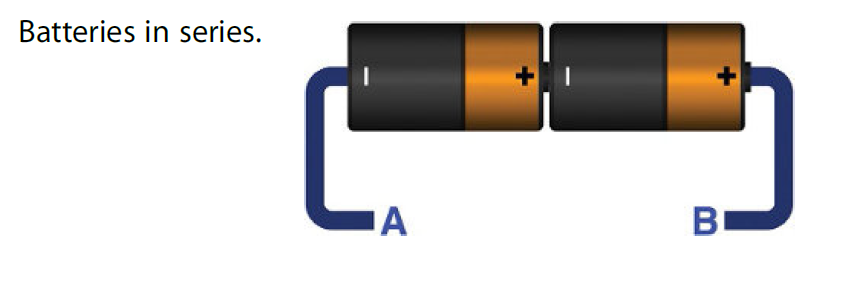
If both batteries are of 2v then we will have total of 4v between point A and B. Batteries can provide a limited amount of current. Since they are in series so if a single battery is providing 0.1A then it will be the total current flowing between A and B. No matter how many batteries are connected in series the current remains same.
What Happens When Two Batteries are Connected in Parallel?
The figure below shows two batteries are connected in parallel.
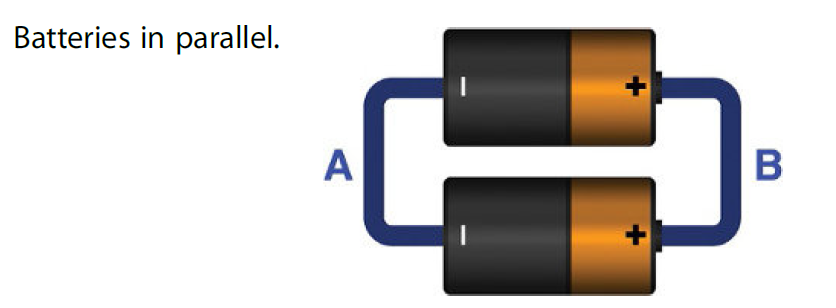
Suppose each battery can provide 0.3V and 0.1A current. The potential (voltage) present between terminals A and B will be 0.3V while the current of these batteries will be added thus giving total current of 0.2A.
Rules for Connecting Batteries
While connecting batteries following rules must be kept in mind.
Do not Invert the Batteries
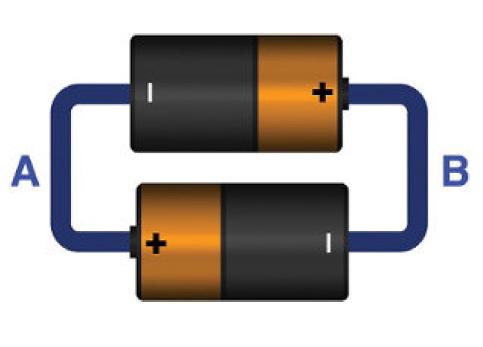
While connecting two batteries or more batteries never ever connect them directly as shown below. It will cause a danger. The other batteries will force current circulation across the inverted one. This will result in excessive heat and may cause explosion.
Avoid Connecting Different Types of Batteries
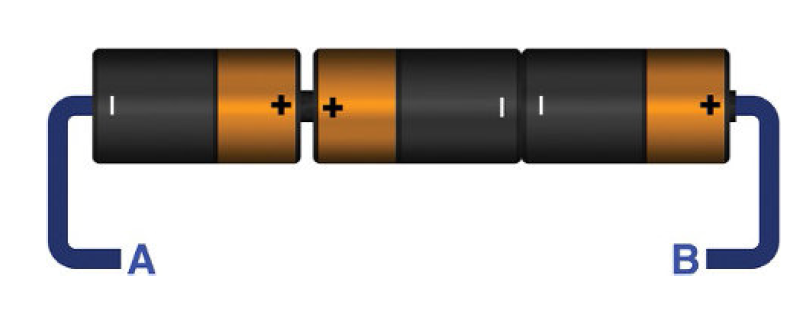
A battery is capable to provide limited amount of current or voltages. If you connect three batteries of different current ratings for example 1A, 2A and 3A in series. Then the largest battery will force to flow 3A of current through each battery. This will cause excess amount of heating and may result in explosion again. The same is true when batteries are connected in parallel.
What Happens When You Short Circuit the Battery?
Never short circuit the batteries like shown below. Current will flow at maximum level thus damaging the both batteries and causing explosion.
Also read here
What are Different Types of an Inductor?
Discuss Certain Phenomenon Where Circuit Theory Fails
what is the electromagnetic induction in electromagnetics?
what are the DC machines?
https://eevibes.com/electronics/electronic-circuits/what-is-the-ohms-law/